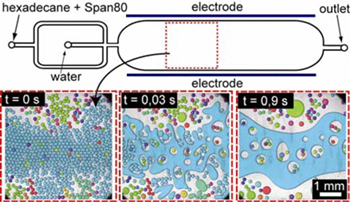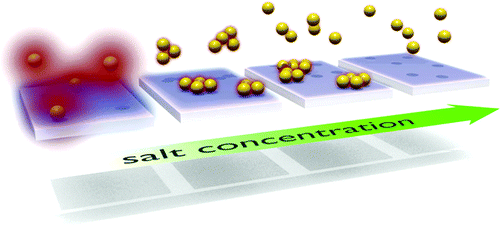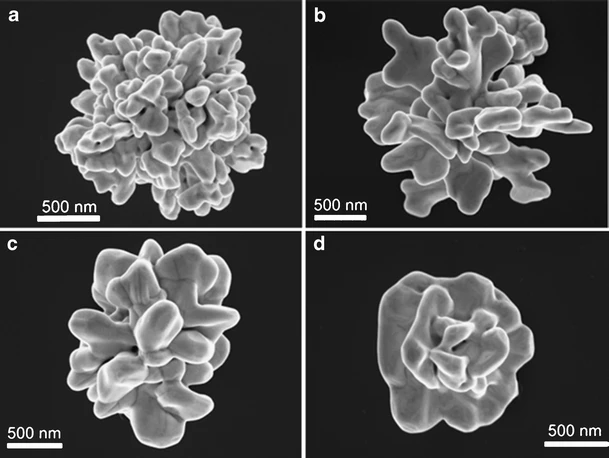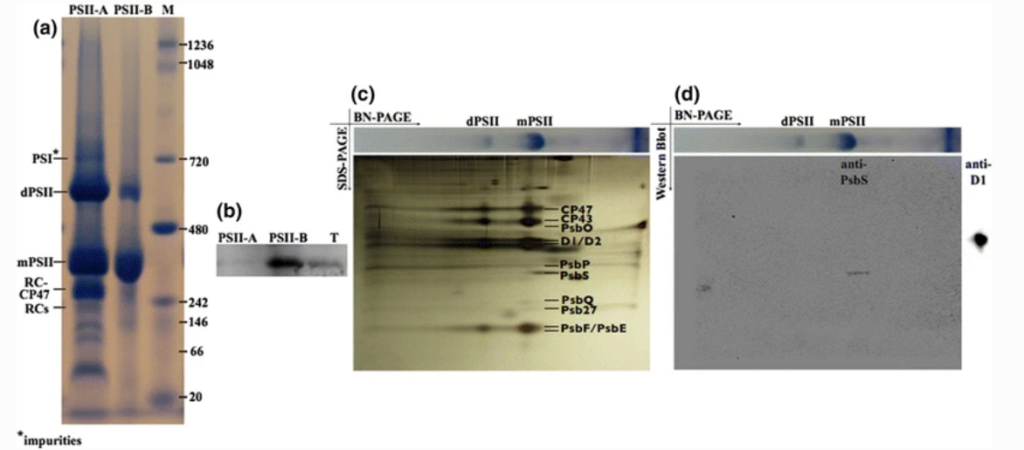
Crossover regime for the diffusion of nanoparticles in polyethylene glycol solutions: influence of the depletion layer
N. Ziębacz, S. A. Wieczorek, T. Kalwarczyk, M. Fiałkowskia and R. Hołyst
Soft Matter, 2011,7, 7181-7186
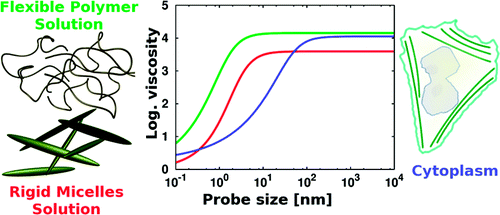
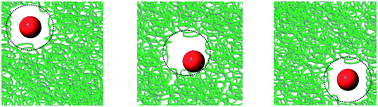
The viscosity in soft matter systems is a scale dependent quantity. In polymer solutions the viscosity of nanoprobes of size R approaches the macroscopic viscosity when R exceeds the radius of gyration of the polymer, Rg. The nano to macroviscosity crossover occurs for R ∼ Rg. Here we analyze diffusion in a polymer (polyethylene glycol) solution of nanoparticles in the crossover regime. We report a scale dependent diffusion coefficient in this regime due to non-uniform viscosity in the depletion layer around particles. The phenomenological scaling of the slow diffusion coefficient as a function of probe size is compared to the same scaling for macroscopic viscosity as a function of polymer size.