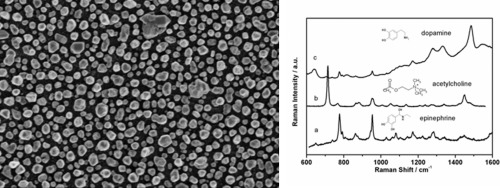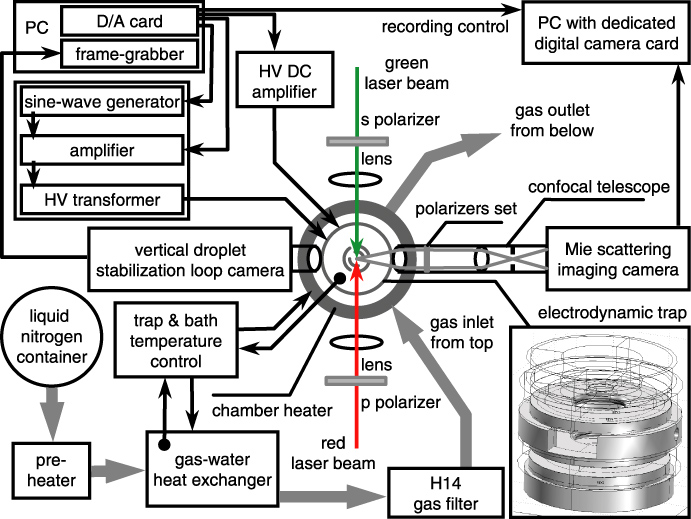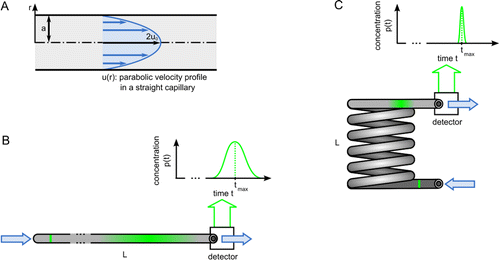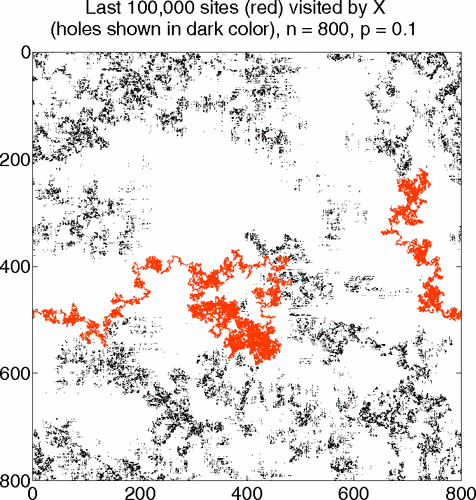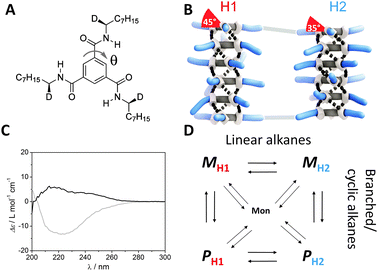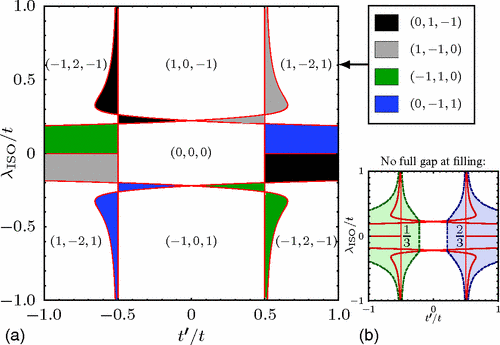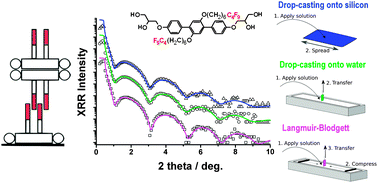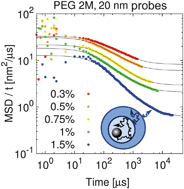
Transport of Mass at the Nanoscale during Evaporation of Droplets: the Hertz–Knudsen Equation at the Nanoscale
M. Zientara, D. Jakubczyk, M. Litniewski and R. Hołyst
J. Phys. Chem. C 2013, 117, 2, 1146–1150
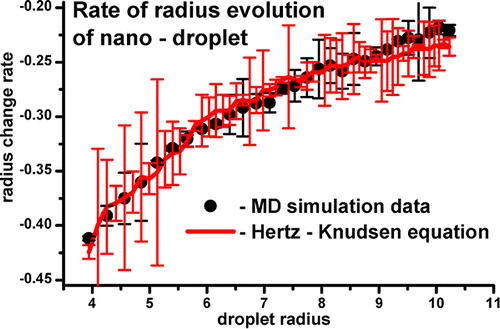
The applicability of the Hertz–Knudsen equation to the evolution of droplets at the nanoscale was investigated upon analysis of existing molecular dynamics (MD) simulations ( Holyst; Phys. Rev. Lett. 2008, 100, 055701; Yaguchi; J. Fluid Sci. Technol. 2010, 5, 180–191; Ishiyama; Phys. Fluids 2004, 16, 2899–2906). The equation was found satisfactory for radii larger than ∼4 nm. Concepts of the Gibbs equimolecular dividing surface and the surface of tension were utilized in order to accommodate the surface phase density and temperature profiles, clearly manifesting at the nanoscale. The equimolecular dividing surface was identified as the surface of the droplet. A modification to the Tolman formula was proposed in order to describe surface tension for droplet radii smaller than ∼50 nm. We assumed that the evaporation coefficient for a system in and out of equilibrium may differ. We verified that this difference might be attributed to surface temperature change only. The empirical dependencies of the evaporation coefficient and the surface tension for a flat interface, of liquid Ar in Ar gas at equilibrium, at the nanoscale, upon temperature was taken from existing MD data. Two parametrizations of the Hertz–Knudsen equation were proposed: (i) one using the off-equilibrium condensation coefficient and the effective density and (ii) another one using the effective density and the temperature at the interface. The second parametrization leads to an approximate solution of the Hertz–Knudsen equation requiring no free parameters. Such a solution is suitable for experimental use at the nanoscale if only the temperature of the droplet (core) can be measured.